Genovior Biotech Corporation (GBC) was founded by Dr. Steve J. P. Hsu in 2015 and has more than 160 employees, of which more than 100 have masters and PhDs in science. It has established advanced factories and R&D bases in Hsinchu (Taiwan), Tainan (Taiwan). It is currently one of the few CDMO companies in Asia that can actually perform and scale up commercial API and injectable, and has certification of PIC/S GMP (cGMP, EU GMP) Part I, II, and III. Rich manufacture experience of Active Pharmaceutical Ingredient (API) and Injectable.
Genovior is a dedicated Ingredient (API) and CDMO focus on process development, scale up, formulation development, and productivity optimization under PIC/S GMP (cGMP, EU GMP) for our customers worldwide. Our one-stop services conveniently provide effective and efficient solution and our non-competition policy provides maximum security and protection in IP for our clients.
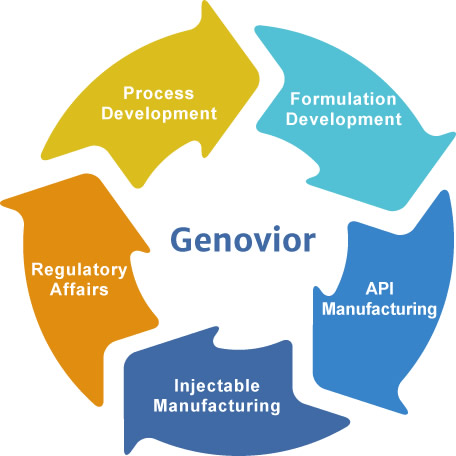
Genovior Biotech Corp. is a vertically integrated contract development and manufacture company, providing following services:
Genovior is a dedicated Ingredient (API) and CDMO focus on process development, scale up, formulation development, and productivity optimization under PIC/S GMP (cGMP, EU GMP) for our customers worldwide. Our one-stop services conveniently provide effective and efficient solution and our non-competition policy provides maximum security and protection in IP for our clients.
Genovior Biotech Corp. is a vertically integrated contract development and manufacture company, providing following services:
- process development of peptide or protein biologics from microbial or human recombinant fermentation processes
- formulation development of injectable
- manufacturing capacities of biologics, lyophilized injectable, and prefilled syringe/cartridge for preclinical, clinical, and commercial uses